Reprinted from Manitoba
Medicine, Vol. 63, No. 3, 1993, pp. 90-91...
Intrathecal baclofen for control of spasticity
in patients with traumatic or inflammatory myelopathy
Patricia Nance1 MD FRCPC, Brian
Schmidt2 MD FRCPC, Derek Fewer3 MD FRCPC
Department of Medicine1,2, Section of Physical Medicine
and Rehabilitation1, Section of Neurology2, Department
of Surgery, Section of Neurosurgery3, University of Manitoba
One of the main inhibitory transmitters
in the central nervous system is gamma aminobutyric acid
(GABA). Baclofen is a GABAb
receptor agonist, resistant to reversal by bicuculline, which
has been used for the treatment of spasticity due to neurological dysfunction
since 1966. Even though baclofen does cross the blood-brain barrier after
oral administration, when given intrathecally baclofen has a much greater
effect and at approximately 1/100th of the oral dose. Intrathecal baclofen
for the treatment of spasticity has been reported in the United States and
Europe.1-4
However, the results of further studies are needed before this treatment can be
made available for general clinical use in Canada. We initiated a prospective
trial of intrathecal baclofen therapy for adults with spasticity due to
spinal cord injury or multiple sclerosis at the Spinal Cord Injury Unit
of the Health Sciences Centre, University of Manitoba in 1990.
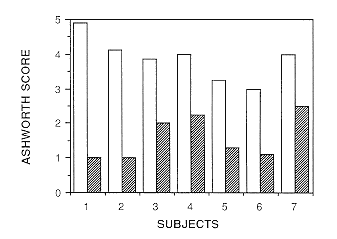 |
| Figure 1 Ashworth score for each subject before intrathecal
baclofen treatment (open bars) compared with scores after treatment (hatched
bars) |
Patients and Study Method
A specialized clinic was established for the assessment and treatment of
spasticity. Over the past two years, we have interviewed and examined 84
patients with spasticity due to spinal cord injury (n=66) or multiple sclerosis
(n=18). There were 19 females and 65 males, ranging in age from 17 to 62
years. Spasticity `severity' was measured in two ways: by using a clinical
scale of leg tone, the Ashworth scale; and by analysis of the pendulum
test. The Ashworth score was determined with the subject in a supine position,
with the limb being moved by the examiner through the available range of
motion to assess four agonist/antagonist muscle groups: hip flexors/extensors,
hip abductors/adductors, knee flexors/extensors and ankle flexors/extensors.
A modified Ashworth scale was used as follows: 1, no increase in tone;
2, slight increase in tone; 3, more marked increase in tone but limb easily
moved; 4, considerable increase in tone, passive movement difficult; 5,
limb rigid in flexion or extension. The pendulum test is the measurement
of the ability of the unsupported leg to swing at the knee.
With regard to the general aspects of treating problematic spasticity,
the majority of the patients seen in the spasticity clinic (74%) had treatable
spasticity-aggravating factors, such as bladder infection, poor urinary
drainage, bladder stones, constipation, leg fractures, skin ulceration,
anal fistula, etc. Oral drugs were prescribed as follows: clonidine 0.05
mg orally, divided into two doses per day (n=26); cyproheptadine 32 mg
orally (n=19), divided into four doses per day; and baclofen 80 mg orally
(n=48), divided into four doses per day. Of the total sample of patients,
10% (eight patients) had severe, intractable spasticity (ie, Ashworth score
greater than 3 and spasm score greater than 2) which persisted throughout
all oral medication treatments. One patient who lives outside of Winnipeg
was excluded. Seven patients passed through the screening phase, which
included the implantation of an intrathecal catheter and temporary port,
the determination of adequate spinal fluid circulation by indium injection
through the port, and the double-blind bolus injection of baclofen. Four
programmable Medtronic pumps and three Infusaid pumps were implanted.
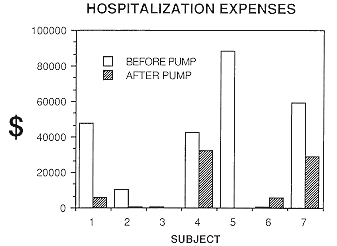 |
| Figure 2 The effects of intrathecal baclofen by implanted
pump infusion on the in-patient hospitalization costs for a one-year period
before treatment (open bars) compared with a one-year period after treatment
(hatched bars) for each subject |
Results
Intrathecal baclofen markedly reduced the Ashworth score, spasms, inhibited
leg reflexes and increased amplitude of knee swing in the pendulum test
(Figure 1). We have not observed changes in respiratory or bladder function
attributable to intrathecal baclofen in these subjects. The complications
we have observed are as follows: failure of the Medtronic one-way valve
(n=1); intrathecal catheter displacement (n=1); weakness of voluntarily
controlled leg muscles (n=1); deep vein thrombosis of the thigh (n=1);
aseptic knee joint effusion (n=1); and aseptic seroma around the pump (n=1).
One subject regained his ability to transfer from his wheelchair independently.
He had skin ulcerations on both ankles for three years which failed to
heal after two attempts at skin grafting before pump implantation, which
healed one month after pump implantation. Another subject who had not walked
for the past five years was able to use a functional electrical stimulation
system to walk short distances with a walker.
A remarkable saving of health care dollars has been realized through
the number of days requiring hospitalization during the post-pump time
period (the amount of time since the subject's pump was installed and the
baclofen dose optimized), compared with the same period of time before
the pump was implanted (Figure 2). The calculated gross amount saved by
the health care budget is $175,804. In addition, Pharmacare plan savings,
calculated on an annual basis for these seven subjects, is $6,195. To project
these savings into the future, the cost of the seven pumps and hospitalization
for their implantation is $128,000.
Conclusion
The majority of patients with spasticity due to injury or multiple sclerosis
can be adequately treated with attention to spasticity aggravating factors
and oral medications. With respect to intrathecal baclofen, conclusions
from our study are preliminary, but it appears to confirm previous reports
regarding the safety and efficacy of intrathecal baclofen in the treatment
of selected patients with severe spasticity. Furthermore, our experience
with these seven subjects suggests that this form of spasticity treatment
is also cost-effective.
Acknowledgements: Dr PW Nance is a Will-to-Win Scholar. Thanks
to Ciba-Geigy for donation of the baclofen for injection. This project
was funded by The Rick Hansen Man-in-Motion Legacy Fund and the Health
Science Centre Foundation, to which the Manitoba Paraplegia Foundation
contributed.
REFERENCES
1. Dralle D Muller H Zierski J Klug N Intrathecal baclofen
for spasticity Lancet 1985 ii
1003 
2. Hankey GJ Stewart-Wynne EG Perlman D Intrathecal
baclofen for severe spasticity Med J Aust 1986 145
465-6 
3. Muller H Zierski J Dralle D Borner U Hoffman O
The effect of intrathecal baclofen in spasticity In Local Spinal Therapy
of Spasticity Muller H Zierski J Penn RD eds Berlin Springer-Verlag 1988
155-214 
4. Penn RD Savoy SM Corcos DC et al Intrathecal baclofen
for severe spinal spasticity N Engl J Med 1989 320
1517-8 
 SCRC
WWW administrator: www@scrc.umanitoba.ca
Reprinted from Manitoba Medicine, Vol. 63, No.
3, 1993, pp. 90-91.
SCRC
WWW administrator: www@scrc.umanitoba.ca
Reprinted from Manitoba Medicine, Vol. 63, No.
3, 1993, pp. 90-91.
Copyright © 1993, Faculty of Medicine, University of Manitoba.
 Back to articles about SCRC
Back to articles about SCRC



![]()
![]()
![]()