Reprinted from
Manitoba Medicine, Vol. 63, No. 3, 1993, pp. 87-89...
Membrane-associated protein factors which inhibit central nervous system regeneration
KW Cheng, PhD
Spinal Cord Research Centre,
Neuroscience Research Program, Department of Physiology,
University of Manitoba
In
higher vertebrates, lesions in the central nervous system (CNS) are
irreversible due to the almost complete lack of regenerative growth from
the injured axons. In obvious contrast, axons of the peripheral nervous
system (PNS) regenerate well. There seem to be no obvious differences,
however, between neurons from the CNS and PNS in their ability to grow
back after injury. Aguayo and
colleagues1 in Montreal
bridged lesions in the spinal cord of rats with a piece from peripheral
(sciatic) nerve; after a short time, the sciatic nerve explant was invaded
from both sides by regenerating neurites that eventually bridged the
injury site, but function was not restored because the neurites stopped
growing after re-entering the spinal cord. These experiments showed that
motor neurons with cell bodies in the spinal cord will re-establish
connections in the periphery but not within the CNS, indicating that
CNS neurons could regenerate in a favourable environment.
The poor regenerative capacity of higher vertebrate CNS might be
explained in one of two ways: either certain growth-promoting substances
are missing, or there are active inhibitors of neurite extension.
For example, Schwann cells in peripheral nerves are surrounded by
basement membranes containing laminin. This extracellular matrix
protein is the most potent substrate for neurite growth, and it acts
synergistically with neurotrophic factors. Since laminin is virtually
absent from the adult CNS of higher vertebrates, it has been argued
that the pattern of expression of laminin might determine whether
regeneration can occur. In addition, Schwann cells in peripheral
nerves produce a variety of neurotrophic factors and even increase this
production after denervation. The hypothesis that there is a difference
in trophic factor production between the PNS and CNS being responsible
for their different regeneration capabilities seems plausible. However,
identified neurotrophic factors such as nerve growth factor, brain-derived
neurotrophic factor, ciliary neurotrophic factor, and fibroblast growth
factor are present in the adult CNS. Furthermore, increases in laminin
as well as neurotrophic factors have been found at central lesion
sites. Why, then, are the re-expressed neurotrophic factors unable to
trigger functional regeneration as in the PNS? Recent studies by several
research groups have suggested that neurite outgrowth may be actively
inhibited by the central glial cells via an inhibitory mechanism.
|
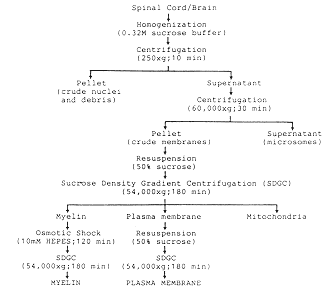
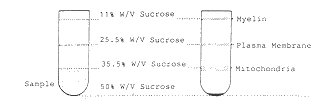
Figure 1 Isolation and characterization of rat
central nervous system (CNS) plasma membrane. A Flow chart
for the isolation of plasma membranes and myelin from adult rat
spinal cord or brain tissues by differential and density gradient
centrifugation. B Fractionation of myelin and plasma membranes
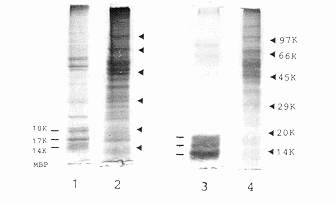
upon sucrose density gradient centrifugation. C Analysis of total
(1,2) and sonication-solubilized (3,4)proteins of purified spinal
cord myelin (1,3) and plasma membranes (2,4) by 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE).
Myelin basic proteins (MBP) 18K, 17K and 14K are the characteristic
proteins of rat CNS myelin
|
Oligodendrocytes in the CNS Inhibit Neurite Outgrowth
Using cell cultures, Schwab and
associates2
at the University of Zurich, Switzerland, observed that cultured neurons
from both the CNS and the PNS extended neurites through a sciatic nerve
explant, but failed to invade a similar explant from optic nerve. The
two explants differed in their myelin producing glial cells, ie,
Schwann cells in the sciatic nerve and oligodendrocytes in the optic
nerve. It is speculated that oligodendrocytes inhibit regeneration
within CNS fibre tracts. Further experiments by co-culturing central or
peripheral neurons with astrocytes, immature oligodendrocytes and mature
oligodendrocytes showed that both CNS and PNS neurons attached to most
cells, except for one type of glial cell. The web of growing neurites
soon formed `windows' around the highly branched mature oligodendrocytes.
When frozen sections of spinal cords were used as culture substrata,
cells adhered mostly to grey matter, indicating that the CNS white
matter is a highly nonpermissive substratum. Similar observations have
been reported by several other investigators. These findings indicate
that oligodendrocytes of the CNS actively inhibit neurite outgrowth by
a contact-mediated mechanism.
Myelin-Associated Neurite Growth Inhibitors
Because oligodendrocytes are the myelin-producing cells of the CNS, myelin
was examined for its property as a neuronal substratum and as a source of
inhibitory components. CNS myelin inhibitory activity is membrane-bound
and associated with the protein fraction of CNS myelin. This inhibitory
substrate activity could be recovered after separation in sodium dodecyl
sulphate polyacrylamide gel electrophoresis (SDS-PAGE)
as two minor
myelin-associated proteins with relative molecular masses (Mr)
of 35 kDa and 250 kDa, now called neurite growth inhibitors NI-35 and
NI-250, respectively.2
These have been shown to be potent inhibitors of neurite outgrowth; an
inclusion of small amounts of these proteins was sufficient to convert
a neutral substrate into a nonpermissive one, and addition of one or
both of these proteins to favourable substrata, such as peripheral
nerve myelin, resulted in inhibition of neurite outgrowth. However,
the inhibitory activity could be neutralized by specific antibodies
raised against these protein inhibitors.
Plasma Membrane Associated Growth Cone Collapsing and Neurite Growth Inhibitory Proteins
Similar, but not identical, membrane-bound
inhibitory proteins have been identified in neural and other tissues, and
reported to induce collapse of axonal growth cones and thus retraction of
neurites. Using time-lapse video analysis, Kapfhammer and
Raper3
of the Max-Planck Institute, Germany, have observed that a few minutes
after contact between the filopodia of a growing retinal axon and a
sympathetic axon, the retinal growth cone thickened and shortened its
filopodia and the neurite retracted. Plasma membranes from chick embryonic
brain have been found to contain components that cause collapse of growth
cones of dorsal root ganglion (DRG) neurons in culture. Furthermore,
membranes prepared from the chick posterior optic tectum have been shown
to collapse growth cones of axons from temporal retina explants. Two
membrane glycoproteins of Mr 48 kDa and 55 kDa from chick
embryonic posterior sclerotome have been observed to induce collapse
of axonal growth cones of DRG neurons in culture. Results of all these
studies by various investigators are consistent with the hypothesis
that growth cone motility is inhibited by specific membrane-associated
proteins in the developing nervous system.
A
|
|
B
|
|
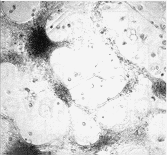
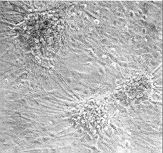
Figure 2 Effects of precoating sonication-solubilized
proteins from adult rat spinal cord plasma membranes on primary cultures of
fetal rat spinal cord neurons. Dissociated fetal rat spinal cord neuronal cells
were plated onto dishes precoated with solubilized plasma membrane proteins
A or control dishes B, and cultured for two weeks in minimal
essential medium containing 5% heat-inactivated horse serum
|
In parallel with these studies of membrane-bound neurite growth inhibitory
proteins, we have recently demonstrated that the plasma membrane of
embryonic chick spinal cord undergoes a developmental transition
from permissive to nonpermissive substrates for neuritogenesis, and
that the transition period occurs around embryonic day 13 of the 21-day
developmental period.4
Cell surface plasma membranes were prepared from homogenates of embryonic
chick spinal cord segments by our established procedure for rat CNS
tissues, as outlined in Figure 1A, and fractionated from myelins by
sucrose density gradient centrifugation (Figure 1B). The plasma membrane
proteins were solubilized by ultrasonication and subjected to an in vitro
assay using clonal NG108-15 cells to monitor permissive and nonpermissive
substrates. The chick spinal cord of early embryonic days (eg, embryonic
day 10) was highly permissive, and the permissiveness decreased with
development as the spinal cord and brain of late embryonic chicks became
highly nonpermissive.4
Recently, in our experimentation on mammalian
CNS, we have observed that plasma membranes from the brain and spinal
cord of newborn and adult rats were highly nonpermissive substrates for
cell adhesion and neurite outgrowth. When cultured on dishes precoated
homogeneously with solubilized proteins from adult rat spinal cord plasma
membranes, primary fetal rat spinal cord neuronal cells remained very
loosely adhesive, forming large `windows' between neuronal aggregates
(Figure 2A), compared with the control of a cell monolayer covering the
whole surface (Figure 2B). This substrate inhibitory activity in plasma
membranes has been observed to be substantially higher than that of the
myelin fraction, suggesting that CNS cell-surface plasma membrane is a
significant cellular source of neurite growth inhibitory proteins. Our
finding of plasma membrane-associated inhibitory proteins appears to
differ from that of Schwab and
associates2
on the myelin-associated
inhibitors NI-35 and NI-250, which are found in the myelin fraction. The
protein content of our rat spinal cord plasma membrane preparation was
characterized by SDS-PAGE to contain major proteins
of Mr 40 to
70 kDa (Figure 1C), significantly different from that of the myelin, which
is characterized by the small Mr 14 to 18 kDa myelin basic
proteins. In addition, from our preliminary data, this plasma membrane
inhibitory principle appears to be an acidic protein of Mr
50 to 70 kDa. However, its molecular structure and biological role in
neuritogenesis remain to be elucidated.
Conclusion
Recent findings indicate that a fine balance between neurite outgrowth
stimulators and inhibitors is essential for the rate and direction
of neurite extension. The plasma membrane is not simply a passive
surface, but functions together with other growth factors and cell
adhesion molecules to serve as a permissive `gating' mechanism for the
function of contact-dependent interactions. Myelin-associated neurite
growth inhibitors, plasma membrane-associated growth cone collapsing
and nonpermissive substrate molecules could play important roles in the
spatial restriction of growth and plasticity in the adult brain and spinal
cord, thereby exerting a stabilizing function for the differentiated
CNS. In view of these recent findings of various neurite outgrowth
inhibitors, it is not difficult to speculate a family or families of
cell type-specific axonal growth-inhibiting regulators. Ongoing research
on molecular mechanisms modulating growth cone behaviour and neurite
extension is essential not only to understand the process of neuronal
differentiation, but also to define the essential conditions necessary
for the regeneration of nerve fibres after spinal cord injury.
REFERENCES
1. David S Aguayo AJ Axonal elongation into peripheral
nervous system `bridges' after central nervous system injury in adult
rats Science 1981214
931-3 
2. Schwab ME Myelin-associated inhibitors of
neurite growth and regeneration in the CNS Trends Neurosci 199013
452-6 
3. Walter J Allsopp TE Bonhoeffer F A common
denominator of growth cone guidance and collapse? Trends Neurosci 1990 11
447-52 
4. Ethell DW Steeves JD Jordan LM Cheng KW
Developmental transition by spinal cord plasma membranes of embryonic
chick from permissive to restrictive substrates for the morphological
differentiation of neuroblastoma X glioma NG108-15 cell Dev Brain Res
1993 72
1-8 
 SCRC
WWW administrator: www@scrc.umanitoba.ca
Reprinted from Manitoba Medicine, Vol. 63, No.
3, 1993, pp. 87-89.
SCRC
WWW administrator: www@scrc.umanitoba.ca
Reprinted from Manitoba Medicine, Vol. 63, No.
3, 1993, pp. 87-89.
Copyright © 1993, Faculty of Medicine, University of Manitoba.
 Back to articles about SCRC
Back to articles about SCRC